Dr. Wozniak's Lab
Research
Our lab is interested in the interactions of the fungal pathogen Cryptococcus neoformans with innate immune cells including dendritic cells and macrophages. Cryptococcus neoformans is the leading cause of fungal meningitis and we are interested in working on immune therapies to prevent or treat this disease. Our current research focuses on innate immune cell interactions with the fungal pathogen Cryptococcus neoformans. For more information on Cryptococcus and the disease it causes, please visit the CDC website.
Current Research
We work on three main projects in the lab:
- Examining the roles of differentially-regulated genes in antifungal activity by macrophage and dendritic cell subsets;
- Examining the mechanism(s) involved in antifungal activity by lysosomal enzymes from DCs, and;
- Examining antifungal potential of novel antifungal compounds.
Cryptococcus neoformans is an opportunistic fungal pathogen that primarily affects immune compromised patients, including those with AIDS and those on immune suppressive therapies to prevent organ transplant rejection. The disease begins as a pulmonary infection that eventually spreads to the central nervous system causing meningitis. Current estimates suggest that approximately 152,000 people are infected with this pathogen each year, and approximately 112,000 die each year due to cryptococcal meningitis (Rajasingham et al., The Lancet, 2022). The initial interaction with the host begins in the lung, and the innate immune cells of the lung (primarily macrophages and dendritic cells) are the front-line of defense against this pathogen.
Role of differentially-regulated genes in antifungal activity of macrophage and DC subsets
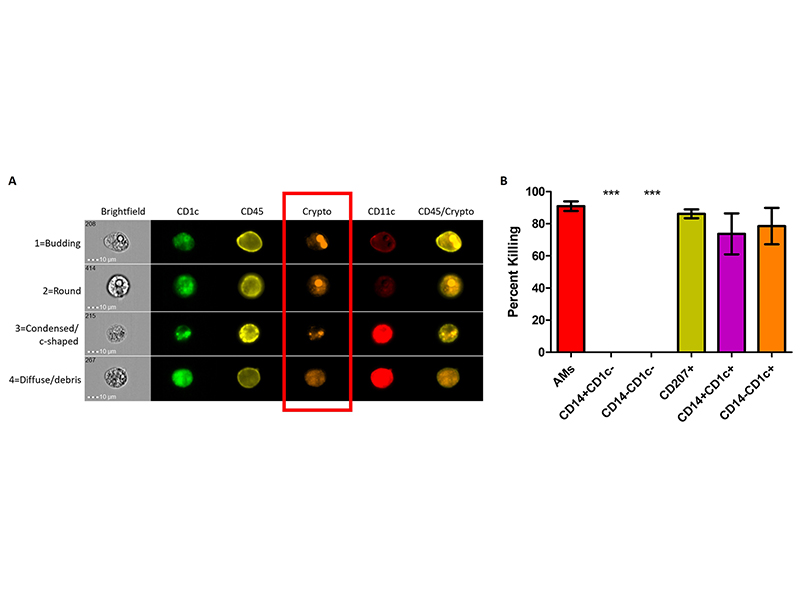
This project examines the macrophage side of the interaction of fungal pathogens, including C. neoformans, with different subsets of macrophages. Many laboratories have examined intracellular growth of C. neoformans and other fungal pathogens inside of macrophages, primarily focusing on cell lines. In primary cells from mice and humans, macrophages subsets exist that interact differently with pathogens. We have shown that the interaction of C. neoformans with two different subsets of human macrophages results in two different outcomes – intracellular growth or intracellular killing. We are currently planning to perform RNA-seq in order to determine differential gene expression in each type of macrophage upon interaction with C. neoformans. These studies can also be applied to other fungal pathogens. The ultimate goal of these studies is to identify mechanisms used by anti-fungal macrophages that could be applied as immunotherapy against fatal C. neoformans infections.
Mechanism(s) by which the lysosomal enzymes have antifungal activity
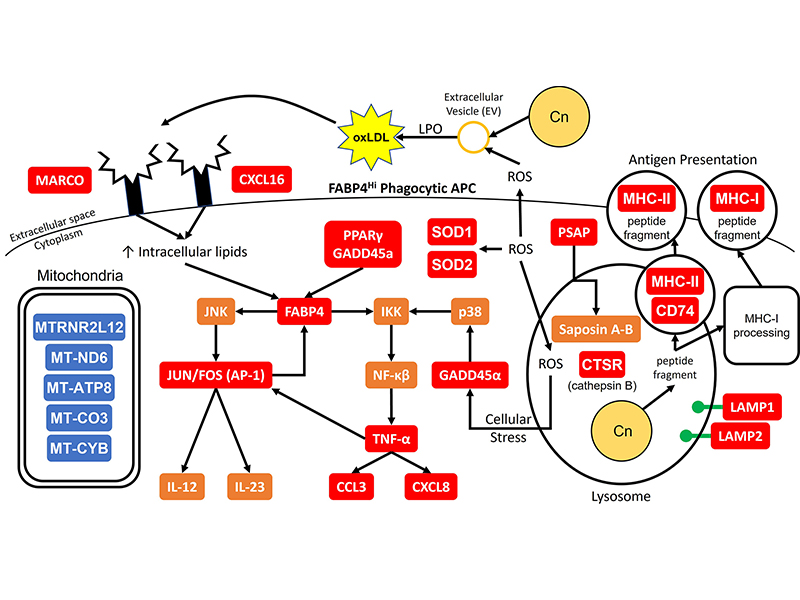
This project examines mechanisms involved with lysosomal degradation of the cryptococcal organism. We have shown that the lysosomal enzyme, cathepsin B, is capable of killing C. neoformans. This happens because of the formation of a hole in the cell wall and leads to osmotic lysis of the organism (Hole et al., Scientific Reports, 2012). We also identified additional antifungal molecules from the DC lysosome (Nelson et al., Scientific Reports, 2021).
Mechanism(s) by which novel antifungal compounds have antifungal activity
These projects examine mechanisms involved antifungal mechanisms of novel antifungal molecules capable of killing C. neoformans. We have several collaborative studies ongoing in the lab, and we have found multiple anti-cryptococcal compounds (Gerasimchuk et al., Molecules, 2022 and unpublished observations). We are exploring the mechanisms using electron microscopy, fluorescent microscopy, and screening of cryptococcal mutant libraries. ). However, we do not understand the mechanism(s) of activity of these compounds.