Research
Our main research focus is on elucidating and characterizing the regulatory role of calcium (Ca2+) signaling in bacterial physiology and virulence.
The primary model that we work with is Pseudomonas aeruginosa, an opportunistic human pathogen that causes severe acute and chronic infections in humans. It is best known as the leading cause of death in cystic fibrosis (CF) patients. Since Ca2+ regulates essential host processes, including hyperinflammatory response to bacterial infection, and accumulates in pulmonary and nasal liquids of CF patients, we predicted that it serves as an environmental signal and enhances the ability of P. aeruginosa to colonize a host and develop infections.
We have characterized the basic prerequisites of intracellular Ca2+ signaling in P. aeruginosa and identified several components of Ca2+ signaling and regulatory network.
Currently, we are working on understanding the functions and the roles of several key proteins transducing Ca2+ signal. They include novel Ca2+-regulated sensor/regulator CarP; EF hand family Ca2+-sensor EfhP; Ca2+ channel CalC; Ca2+-dependent two-component regulatory system CarSR; and Ca2+- and phosphate-regulated protein PcrP. We are interested to learn the molecular details of how these proteins sense Ca2+ and control numerous Ca2+ responses in P. aeruginosa.
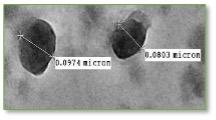 We also interested in the mechanisms of cellular Ca2+ homeostasis and its role in calcium deposition, cell-cell communication, and interactions with host. We have shown that P. aeruginosa is able to deposit extracellular Ca2+, which requires at least one of six functional carbonic anhydrases, may trigger soft
tissue calcification in a host, and thus contribute to P. aeruginosa pathogenicity. We focus on characterizing carbonic anhydrases, their physiological
roles and potential for inhibition.
We also interested in the mechanisms of cellular Ca2+ homeostasis and its role in calcium deposition, cell-cell communication, and interactions with host. We have shown that P. aeruginosa is able to deposit extracellular Ca2+, which requires at least one of six functional carbonic anhydrases, may trigger soft
tissue calcification in a host, and thus contribute to P. aeruginosa pathogenicity. We focus on characterizing carbonic anhydrases, their physiological
roles and potential for inhibition.
Our most recent focus is on the Ca2+-dependent mechanistic aspects of P. aeruginosa interactions with host cells during cell adhesion and internalization. We are utilizing dual RNA-seq approach followed by genetic, biochemical and functional studies to build a new understanding of how Ca2+ signaling empowers P. aeruginosa invasion and adaptation to the host.

