Research
Binary Zintl Anions
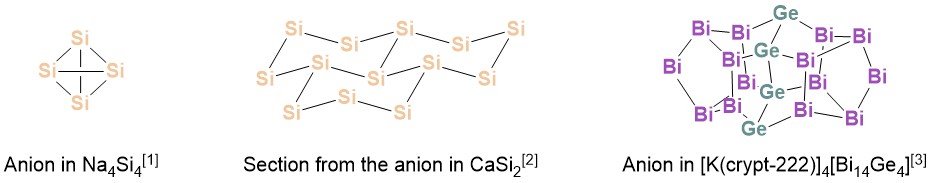
Zintl compounds, featuring polyanions formed by elements from group 14 to 17, exhibit fascinating structural diversity (image) and thus find application in many areas, especially in materials science.[4] Tetrahedral [Tt4]4− (Tt = tetrel / group 14 element) anions (image left) are interesting due to their similarity to white phosphorus, which exhibits versatile reactivity. However, high atomic charges and poor solubility prevented such compounds to be used as substrates within a broad scope. Partial replacement of the negatively charged group 14 elements with neutral group 15 elements provides a way to decrease the overall charge, increase the solubility, and thus make those compounds more readily available for follow-up chemistry.[5] Since established synthetic strategies are typically “top-down” methods based on breaking down larger structures, they lack precision concerning size control and composition of the anions. On the other hand, “bottom-up” approaches, which are based on the systematic design of Zintl compounds from small building blocks and allow for control of the resulting cluster sizes and shapes, are rare. Thus, we are developing new "bottom-up" approaches towards binary Zintl anions that have not yet been accessible by "top-down" methods.
Perfluoropolysilapolyhedranes
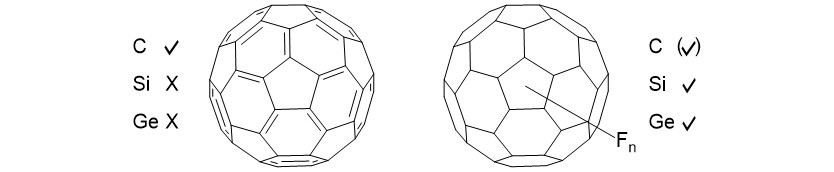
While carbon-based fullerenes are extremely stable, all efforts to synthesize analogues based on the heavier homologues were futile to date (image left). However, there is an intense interest to study such compounds and several strategies to stabilize a silicon framework have been proposed, one of them being perfluorination (image right).[6,7] Despite the interest in this class of compounds, the entire field of perfluorinated silicon cages is virtually unexplored and mainly subject to theoretical studies, although a large number of them is predicted to be stable.[6] This project aims for the development and characterization of the first perfluorinated silicon cage compounds to provide access to a new compound class.
References
[1] E. Hohmann, Z. Anorg. Allg. Chem. 1948, 257, 113; G. Kliche, M. Schwarz, H.-G. von Schnering, Angew. Chem. 1987, 99, 350.
[2] J. Evers, J. Solid State Chem. 1979, 28, 369.
[3] R. J. Wilson, S. Dehnen, Angew. Chem. Int. Ed. 2017, 56, 3098.
[4] F. Laves, Naturwissenschaften 1941, 29, 244; S. C. Sevov, J. M. Goicoechea, Organometallics 2006, 25, 5678; L. Guggolz, S. Dehnen, Chem. Eur. J. 2020, 26, 11819; S. Scharfe, T. F. Fässler, Phil. Trans. R. Soc. A 2010, 368, 1265; S. Gärtner, N. Korber in Comprehensive inorganic chemistry II. From elements to applications (Ed.: J. Reedijk), Elsevier, Amsterdam, 2013, pp. 251–267; D. Beretta, N. Neophytou, J. M. Hodges, M. G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti, W. Tremel et al., Mater. Sci. Eng., R 2019, 138, 100501; L. Zheng, W. Li, C. Sun, X. Shi, X. Zhang, Y. Pei, J. Alloys Compd. 2020, 821, 153497; O. P. E. Townrow, C. Chung, S. A. Macgregor, A. S. Weller, J. M. Goicoechea, J. Am. Chem. Soc. 2020, 142, 18330.
[5] B. Weinert, S. Dehnen in Clusters: Contemporary Insight in Structure and Bonding, pp. 99–134.
[6] M. Anafcheh, R. Ghafouri, Phys. E (Amsterdam, Neth.) 2013, 48, 13.
[7] L. Wang, D. Yang, Mol. Simul. 2010, 36, 493.